Clinical trial underway at Baylor Scott & White on lab-manufactured COVID-19 antibodies
Clinical trial underway at Baylor Scott & White on lab-manufactured COVID-19 antibodies
The Dallas research institute enrolled the first patients in the world to test a new type of manufactured antibodies.
DALLAS - A new clinical trial that is part of Operation Warp Speed is now underway at Baylor Scott & White.
The research institute enrolled the first patients in the world to test a new type of manufactured antibodies.
Baylor Scott & White Research Institute became the first site in the world to begin testing a new type of antibody manufactured in a lab on hospitalized patients.
And while the trial is part of Operation Warp Speed, the director says there will be no shortcuts to getting answers.
Dr. Uriel Sandkovsky is leading the new clinical trial at Baylor Scott & White in partnership with the National Institute of Health.
“To be able to offer those who walk through the door hope, I think that is the most important part,” he said.
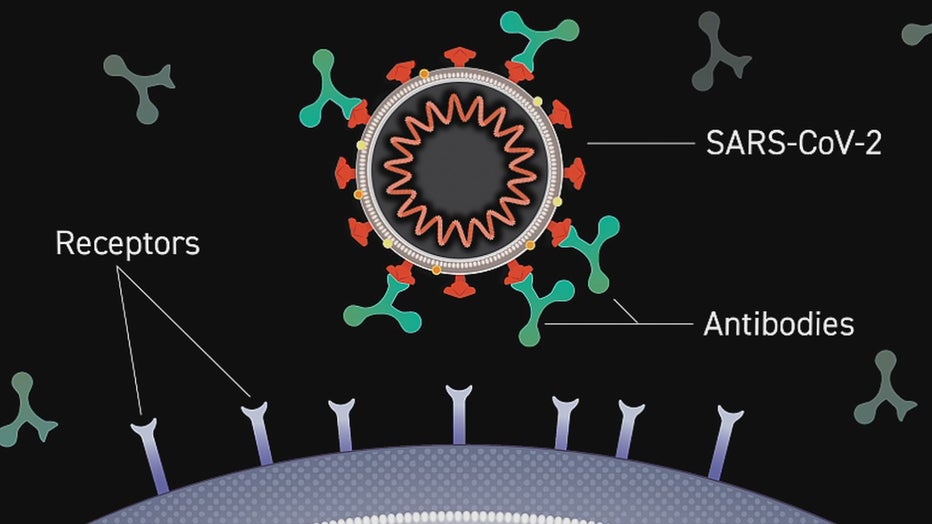
The trial tests an antibody manufactured in a laboratory for hospitalized patients. A monoclonal antibody attaches to the coronavirus, blocking its entry on a human cell.
Unlike most trials, in this case, if the antibody medication is not working, Baylor Scott & White can adapt quickly to cut red tape.
“We are allowed to bring in another treatment in the trial,” Dr. Sandkovsky said. “If an antibody doesn't work we can try an antibody from a different company to use for our patients.”
Patients in the trial will not know if they are given a monoclonal antibody or a placebo. All patients will still be given the antiviral Remdesivir, the standard hospital treatment right now for COVID-19.
“We know 100 percent dealing with people not guinea pigs. The intent is to help people,” the doctor said.
Dr. Sandkovsky says whether a medication is found that saves lives in Dallas or not, he knows pieces to the treatment puzzle will be found.
“It’s a team effort around the world. We are a piece of that. We have to be humble,” he said.
The only patients eligible for the clinical trial are those who require hospitalization. Five patients have volunteered to be part of the trial in Dallas so far.

